
The Medicines and Healthcare products Regulatory Agency (MHRA) has started the rolling review process of a coronavirus vaccine being developed by Moderna in the US, the company has announced.
This means the MHRA will review data as it becomes available from ongoing studies, before a formal application for approval is submitted.
By reviewing the data in this way, the regulator can reach an opinion sooner on whether the medicine or vaccine should be licensed in the UK.
In a statement, Moderna said: “This announcement follows positive results from a pre-clinical viral challenge study of mRNA-1273 and the positive interim analysis of the Phase 1 study of mRNA-1273 in adults (ages 18-55 years) and older adults (ages 56-70 and 71+) published in the New England Journal of Medicine.”
Stephane Bancel, chief executive officer of Moderna, said: “We appreciate the collaboration we have had to date with regulatory authorities around the world, and the process established by the MHRA to address this ongoing public health emergency.
“This is a great example of what’s being done to support efforts to deliver a safe and effective vaccine to UK citizens as safely and efficiently as possible.”
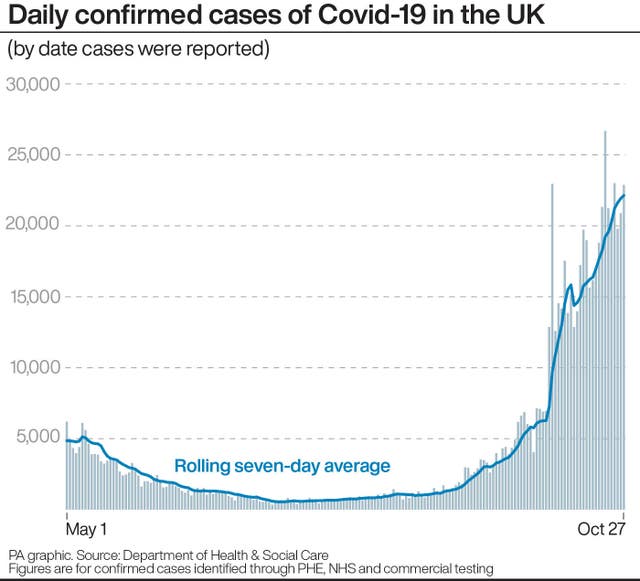 (PA Graphics)
(PA Graphics)
Interim analysis of phase one of the trial showed the vaccine, mRNA-1273, was generally well-tolerated across all age groups and induced rapid and strong immune responses against SARS-CoV-2, the virus which causes Covid-19.
The vaccine is in phase three trials with 30,000 participants.
Dr Christian Schneider, interim chief scientific officer at MHRA, said: “We are ready to prioritise and carry out a rolling review of data for candidate Covid-19 vaccines.
“We invite any company to submit licence applications for Covid-19 vaccines they anticipate requiring approval in the early part of 2021 to us as soon as they have relevant information.”
Stephen Evans, professor of pharmacoepidemiology at London School of Hygiene and Tropical Medicine, said: “Most major regulators have said that they will start to assess early data from the first phases of development of a vaccine.
“Though assessment of the early data has begun, it does not guarantee that this or any other vaccine will be available in 2020, but if the phase three results are good, then this unusual if not unprecedented approach will allow the vaccine to be available more quickly.
“It is entirely possible that other companies have been doing the same thing, either through the UK or other countries’ regulatory system, but they have possibly not felt it necessary to announce it publicly.”


Why are you making commenting on The Herald only available to subscribers?
It should have been a safe space for informed debate, somewhere for readers to discuss issues around the biggest stories of the day, but all too often the below the line comments on most websites have become bogged down by off-topic discussions and abuse.
heraldscotland.com is tackling this problem by allowing only subscribers to comment.
We are doing this to improve the experience for our loyal readers and we believe it will reduce the ability of trolls and troublemakers, who occasionally find their way onto our site, to abuse our journalists and readers. We also hope it will help the comments section fulfil its promise as a part of Scotland's conversation with itself.
We are lucky at The Herald. We are read by an informed, educated readership who can add their knowledge and insights to our stories.
That is invaluable.
We are making the subscriber-only change to support our valued readers, who tell us they don't want the site cluttered up with irrelevant comments, untruths and abuse.
In the past, the journalist’s job was to collect and distribute information to the audience. Technology means that readers can shape a discussion. We look forward to hearing from you on heraldscotland.com
Comments & Moderation
Readers’ comments: You are personally liable for the content of any comments you upload to this website, so please act responsibly. We do not pre-moderate or monitor readers’ comments appearing on our websites, but we do post-moderate in response to complaints we receive or otherwise when a potential problem comes to our attention. You can make a complaint by using the ‘report this post’ link . We may then apply our discretion under the user terms to amend or delete comments.
Post moderation is undertaken full-time 9am-6pm on weekdays, and on a part-time basis outwith those hours.
Read the rules here